Introduction: Chronic Lymphocytic Leukemia (CLL) is characterized by the accumulation of mature-appearing malignant lymphocytes (CLL B-cells) in the blood, marrow, lymph nodes, and spleen. Despite improved outcome with the introduction of novel BCR and BCL-2 inhibitors, disease progression is still a therapeutic challenge from either differential responses or acquired drug resistance. Recent studies in CLL reported alterations of the epigenetic landscape as well as mutations of genes encoding key chromatin machineries. These aberrant chromatin structures may provide novel therapeutic targets for CLL. Here, we identify aberrant chromatin features in CLL B-cells as novel therapeutic targets.
Methods: Histones were extracted by acid from B cells derived from 10 random selected CLL patients and 10 normal donors and histone modifications were checked by western blot. For ChIP-seq study, published H3K27me3 ChIP-seq data (GSE113336) were downloaded from and analyzed (Control samples, n= 6; CLL samples, n=16). Gene ontology analysis used the Panther Classification System. Cell Survival was determined by CellTiter 96® AQueous Assay (Promega).
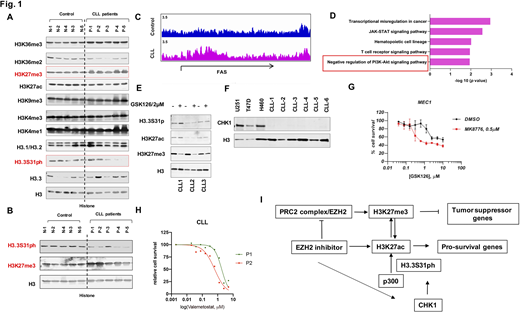
Results: While most histone modifications do not vary between CLL and controls, H3K27me3 and H3.3S31ph are increased and decreased respectively, albeit variably, in CLL B-cells (Fig1. A and B). Notably, the low level of H3.3S31ph was observed in a subset of samples (7 of 10 CLL samples tested). To further investigate the biology and role of H3K27me3 in CLL, we analyzed its genome-wide distribution by chromatin immunoprecipitation followed by sequencing (ChIP-seq). Our analysis showed that the genes with increased H3K27me3 occupancy were mostly enriched in tumor suppression pathways (e.g., negative regulation of PI3K-Akt pathway) or down-regulated genes in CLL such as genes involved in the pro-apoptotic pathway (FAS) (Fig1. C and D). These results suggested that high enrichment of H3K27me3 may regulate the expression of these genes, contributing to CLL survival. H3K27 methylation, an important suppressive histone modification that is associated with transcription repression, is catalyzed by Polycomb Repressive Complex 2 (PRC2). Therefore, inhibition of Enhancer of Zeste Homolog 2 (EZH2), the catalytic subunit of PRC2, could be explored as a therapy approach in CLL. However, feedback activation of H3K27 acetylation (H3K27ac) can promote expression of pro-survival genes that confers EZH2 inhibitor (EZH2i) resistance, which limits its use in human malignancy. Thus, the epigenetic determinants that reliably overcome EZH2i resistance or sensitize cells to EZH2 inhibition have yet to be identified. As we observed that the CLL B-cells in a subset of CLL patients have low levels of H3.3S31ph, and a recent study showed the importance of H3.3S31ph for the enzymatic activity of p300 to acetylate H3 at lysine 27(Martire S et al., Nat Genet. 2019), we assessed the role of H3.3S31ph in the process of EZH2 inhibitor-mediated H3K27ac. Our results showed that inhibition of H3.3S31ph by CHK1 inhibitor MK-8776 abolished the activation of H3K27ac by EZH2i in MEC1 cells, which represents the patients who have CLL cells with relatively high level H3.3S31ph. However, we did not see a major increase of H3K27ac and H3.3S31ph in primary CLL B-cells with EZH2 inhibition (Fig. 1E), consistent with the relatively low expression of CHK1 protein in these cells (Fig. 1F).
Because our data shows the requirement of H3.3S31ph in H3K27ac activation by EZH2 inhibition, we next tested if H3.3S31ph inhibition could overcome H3K27ac induced EZH2 inhibition resistance. We found that suppression of H3.3S31ph by CHK1 inhibitor MK-8776 sensitizes the CLL-like line MEC1 to EZH1/2 inhibition (Fig. 1 G). We then showed that an EZH2 inhibitor, Valemetostat, reduce the survival of the primary CLL B-cells (Fig. 1 H). These results suggest that the low level of H3.3S31ph may provide a therapeutic opportunity for CLL treatment with EZH inhibition.
Conclusion: In summary, we have elucidated how epigenetic features in leukemic CLL B-cells (H3K27me3 and H3.3S31ph), can provide novel treatment targets for CLL (Fig. 1 I). Moreover, this study may provide a proof of concept to develop new treatment strategies based on epigenetic vulnerabilities in other hematological malignancies.
Parikh:GlaxoSmithKline: Honoraria; MorphoSys: Research Funding; Genentech: Honoraria; Ascentage Pharma: Research Funding; AbbVie: Honoraria, Research Funding; TG Therapeutics: Research Funding; Janssen: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding; Verastem Oncology: Honoraria; Merck: Research Funding. Kenderian:Kite: Research Funding; MorphoSys: Research Funding; Tolero: Research Funding; Humanigen: Consultancy, Patents & Royalties, Research Funding; BMS: Research Funding; Gilead: Research Funding; Juno: Research Funding; Lentigen: Research Funding; Mettaforge: Patents & Royalties; Novartis: Patents & Royalties, Research Funding; Torque: Consultancy; Sunesis: Research Funding. Ding:Beigene: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Membership on an entity's Board of Directors or advisory committees; alexion: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Research Funding; DTRM: Research Funding; Abbvie: Research Funding. Braggio:DASA: Consultancy; Bayer: Other: Stock Owner; Acerta Pharma: Research Funding. Kay:Oncotracker: Membership on an entity's Board of Directors or advisory committees; Juno Theraputics: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees; Morpho-sys: Membership on an entity's Board of Directors or advisory committees; Cytomx: Membership on an entity's Board of Directors or advisory committees; Agios Pharma: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Sunesis: Research Funding; MEI Pharma: Research Funding; Abbvie: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tolero Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squib: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.